
Feature Story
New guidance on ethical HIV prevention trials published
27 January 2021
27 January 2021 27 January 2021UNAIDS and the World Health Organization have published updated guidance on ethical considerations in HIV prevention trials. The new guidance is the result of a year-long process that saw more than 80 experts and members of the public give inputs and is published 21 years after the first edition appeared.
“UNAIDS is committed to working with the people and populations affected by HIV, promoting and protecting their rights,” said Peter Godfrey-Faussett, UNAIDS Science Adviser. “This guidance sets out how to carry out ethical trials on HIV prevention while safeguarding the participants’ rights during scientific research and promoting the development of new HIV prevention tools.”
With 1.7 million people becoming newly infected with HIV in 2019, there is still an urgent need to develop new ways of preventing HIV and make them available so that people can protect themselves from the virus. While new methods of preventing HIV have been developed over the past few years, for example pre-exposure prophylaxis, taken orally, in the dapivirine ring or in long-acting cabotegravir injections, the demand for easy-to-use and effective HIV prevention tools remains strong.
However, the need to develop new HIV prevention methods needs to be balanced with the need to protect the people who participate in scientific studies of the safety and efficacy of the prevention tools.
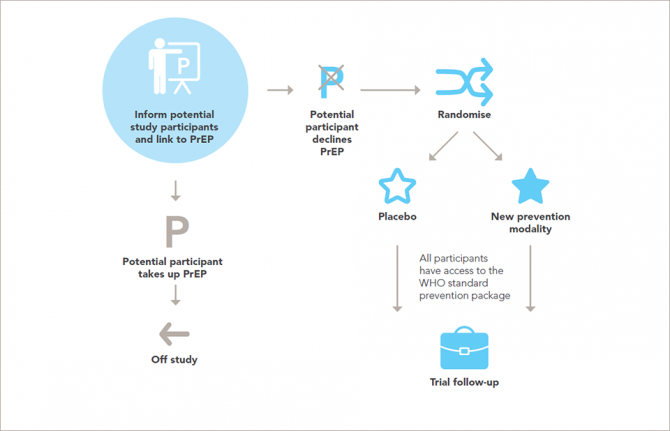
Research on people is governed by a well-established framework of ethical standards. The new report sets out in 14 guidance points the ethical standards for research on HIV prevention and upholds and explains the universal principles of ethics for research involving people in ways that are relevant to the participants and to developments in research for HIV prevention.
“The World Health Organization must ensure that policymakers and health implementers keep ethics at the heart of their decision-making. This collaboration with UNAIDS in convening a wide range of stakeholders for the review is a model for the future development of ethics guidance,” said Soumya Swaminathan, Chief Scientist at the World Health Organization.
The ethical considerations surrounding HIV prevention research are complex. Research must be conducted with the populations for which the new methods might have the most impact—such as key populations and adolescent girls and young women in locations where there is a high incidence of HIV—but members of those populations often live in situations that make them vulnerable to discrimination, incarceration or other harm, which can limit their participation in research and makes ethical research more challenging. The updated guidance seeks to set out how to ethically incorporate the needs of the people who could most benefit from HIV prevention research.
The updated guidance includes a number of key revisions to the previous edition. The importance of community members being involved at all stages of research projects is highlighted—there must be an equal partnership among research teams, trial sponsors, key populations, potential participants and the communities that live in settings where trials are taking place.
The issue of fairness, with an inclusive selection of study populations without arbitrary exclusion on the basis of characteristics such as age, pregnancy, gender identity or drug use, is emphasized. The guidance also underlines contexts of vulnerability—people and groups should not be labelled as vulnerable, but rather the emphasis should be on the social or political contexts in which people live that may render them vulnerable.
That researchers and trial sponsors should, at a minimum, ensure access to the package of HIV prevention methods recommended by the World Health Organization for every participant throughout the trial and follow-up is set out in the updated guidance, along with the need for post-trial access by participants to products that are shown to be effective.
“This revised guidance will support all stakeholders in designing and conducting ethically and scientifically sound HIV prevention trials that advance the AIDS response towards the goal of zero new HIV infections,” added Mr Godfrey-Faussett.
This guidance document is also available in Portuguese.



