Feature Story
Responsible journalism honoured in Bangladesh
14 March 2008
14 March 2008 14 March 2008
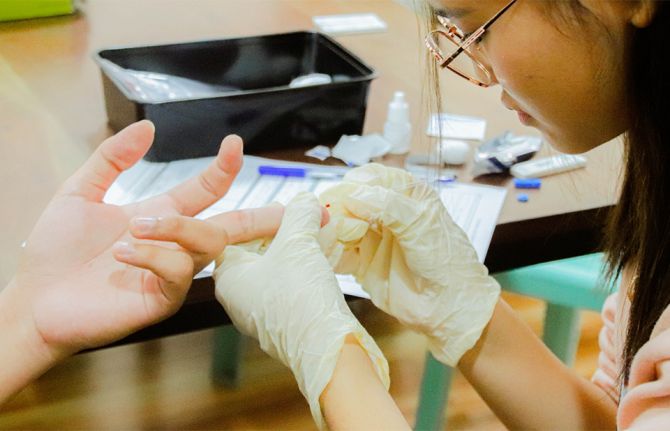
Award winners of the UNAIDS Bangladesh Media Awards, 13 March 2008.
Credit: UNAIDS
Four outstanding journalists were honoured on 13 March in Bangladesh for their exceptional contribution to the AIDS response through journalism at the first ever UNAIDS Bangladesh Media Awards.
There is a low HIV prevalence in Bangladesh. As a consequence, there is a relatively low level of knowledge and awareness about the disease among the general public and people living with HIV experience considerable social stigma and discrimination.
At the backdrop of the context described above, UNAIDS office in Bangladesh, in collaboration with the National Media Forum on HIV and AIDS (APLF initiative), created a platform from which ethical and responsible journalism is encouraged. The awards went to those who promoted the rights of most at risk populations and people infected and affected by HIV.
“The media in Bangladesh and elsewhere have a great responsibility to not contribute to a culture of discrimination by perpetuating stereotypes,” said UNAIDS Country Coordinator, Dan Odallo. “These award winners offer excellent examples of sensitive and informed journalism.”
First place for National Print media award was won by investigative journalist Ms. Qurratul-Ain-Tahmina for a three-part series called “Bangladesh at risk of AIDS”. Qurratul-Ain-Tahmina has been highlighting human rights and other important social issues in the media for several years.
Another winner was a popular national radio station Radio Today who took first prize in the Electronic media category for a catchy public service announcement on an HIV prevention broadcast.

A panel of guests addressed the awards ceremony. Credit: UNAIDS
The Directorate General Health, Dr S. M. Mustafa Anower highlighted the significance of the media’s role in the AIDS response. “60% of people in Bangladesh receive most of their news and information through print and electronic media, therefore media is a most powerful vehicle in the fight against HIV and AIDS,” he said.
Honourable Advisor, Ministry of Health & Family Welfare, Dr A M M Shawkat Ali, encouraged more media training for journalists who in rural areas to ensure that responsible journalism reaches everyone in Bangladesh.
“Awareness always needs to be created at the grass roots level with simple and easy to understand messages,” he said. With a low adult literacy rate of 53%, this is considered particularly important in Bangladesh.
APLF Champion for AIDS, singer Runa Lailor, congratulated the Standard Charted Bank for their financial support of the award and their partnership in the fight against AIDS. She expressed the hope that this social responsibility initiative will encourage other journalists to increase the frequency and sensitivity of coverage of AIDS issues in the media.
UNAIDS Bangladesh plans to hold the Media Awards annually.
Responsible journalism honoured in Bangladesh
External links:

Feature Story
Much progress to report: UNGASS 2008
12 March 2008
12 March 2008 12 March 2008
It was already a cold night, in Dushanbe Tajikistan and it was made worse that the heating to building was cut off. The indoor temperature had dropped to -40 decrees Celsius. It was however 31 January 2008 the deadline for countries to send their responses for the UNGASS reporting system. Undeterred by the freezing temperatures, the government staff tasked on this project continued to work in order to meet the deadline.
Every two years, in compliance with the Declaration of Commitment on HIV/AIDS signed by UN member states in June 2001, countries report to UNAIDS on their progress made in the response to the AIDS epidemic. This year, countries and individuals showed extraordinary personal commitment to this reporting process.
Extraordinary lengths to submit the reports on time
While post-election civil unrest beset Kenya, civil society and staff of government ministries risked injury by continuing to travel to their offices during street rioting in order to complete their report.
That so many countries have submitted their progress reports, and in some cases gone to such exceptional lengths to do so, is a testament to how seriously the member states are taking their responsibility of reporting progress towards the Declaration of Commitment unanimously adopted in the 2001 UN General Assembly Special Session on HIV and AIDS.
Submission rates higher
As of 7 March 2008, 147 out of 192 countries had submitted their reports; this is a large increase from the 2006 number of 115 countries out of a 189.
Submission rates stand significantly at 100% of the Caribbean, 95% of Eastern Europe and Central Asia, 95% of Latin America and 94% of sub-Saharan Africa.
However, by the same date only 50% of North America, 53% of Western and Central Europe and 50% of North Africa and Middle East had submitted their reports. East Asia reporting stands at 60% and Oceania 57%.
The high submission rate in several regions is indicative of how many countries are prepared to be publicly accountable. The UNAIDS secretariat publishes these reports on the UNAIDS web site exactly as submitted by the country, without editing or other alteration. Far from seeing the reporting process as a bureaucratic exercise, instead it is being seen as an opportunity to make a public statement about the country’s commitment and to be compared side by side with other countries.
“The approach to this year’s reporting process was unprecedented in terms of its coordination and participation and the results speak for themselves,” said UNAIDS Chief of Monitoring and Evaluation Division, Dr Deborah Rugg.
“Governments, civil society, UNAIDS cosponsors and the Secretariat all pulled together in a concerted way to ensure that the country reports were more comprehensive than ever. It is a credit to the commitment of all involved and an expression of the exceptional response that is required by this epidemic.”
Civil society participation
The participation of civil society is an essential part of the reporting process and they have a particularly important role to play in the compilation of the National Composite Policy Index (NCPI) indicators.
UNAIDS has engaged a consortium of civil society organizations led by the International Council of AIDS Service Organizations and the International Women's Health Coalition (ICASO and IWHC) to provide support to civil society organizations in national reporting. This support has been made available at the regional level, through a number of networks working with in partnnership with ICASO and IWHC.
In many countries civil society has been engaged in the national reporting process, providing inputs at different levels including providing complimentary or qualitative data to supplement national reports, engaging in national reporting workshops and producing shadow reports. A shadow report may be submitted by civil society where civil society was not adequately included in the national reporting process or where governments do not submit a country progress report.
There has been a significant drop in number of shadow reports submitted to UNAIDS, reflecting the substantial efforts in many countries to increase the engagement of civil society in national reporting processes. These reports sometimes reflect the perspectives of marginalized constituencies who, for a variety of reasons, can find it difficult to gain access to more formal reporting processes.
The shadow reports produced by civil society organizations are available on the ICASO web site.
Investment yields results
UNAIDS Secretariat also increased investment into the training process by organizing regional workshops for government monitoring and evaluation officers and providing 25 expert consultants to travel to countries to assist them in their reporting process.
Reconciled data
This year’s process was also very successful in terms of data reconciliation. Where there were discrepancies in country data reported for indicators as part of the UNGASS process and as part of reporting to other UN agencies, (for example mother-to-child transmission numbers or treatment numbers), joint UN teams worked closely together in countries to reconcile these numbers by analysis and by examination of the methodologies used, and agreeing on the most valid indicator value.
This was the first time where divergences in the data where identified at the global level and then reconciled at the country level in a collaborative way. The initiative was led by the UNAIDS Secretariat who convened a forum with five cosponsors and partners, including WHO, UNICEF, PEPFAR, MEASURE/DHS, and the Global Fund.
Countries reported that the process was a catalyst promoting very healthy dialogue between agencies and reinforced the country ownership principle.
Global Response Database (GRD)
UNGASS indicator data are submitted by each country using a software tool called the Country Response Information System (CRIS). UNAIDS has now developed a large global database called the Global Response Database (GRD). The GRD allows better analysis of the global response, country by country and region by region, and for the first time allows multi-year trend for some of the UNGASS indicators and disaggregation by gender and age.
In addition to allowing data to be imported from other sources, the GRD will enable the future sharing of UNGASS data with UNAIDS Cosponsors such as UNICEF and WHO and also the UNAIDS Regional Support Teams.
Use of the UNGASS data
The UNGASS indicators will be used to monitor the progress towards achieving Universal Access to prevention, treatment and care in 2010 and eventually in reaching the Millennium Development Goal of arresting and halting the spread of HIV by 2015.
The UNGASS data will form the basis of the UN Secretary-General’s report to the General Assembly in June as well as the 2008 Report on the global AIDS epidemic to be launched at the Mexico City International AIDS Conference 3-8 August 2008.
As many improvements have been seen in this year’s process in terms of the quality of data, civil society participation, number of reports and the data analysis capacities we can look forward to getting a clearer picture of the world’s response to the epidemic.
An improved reporting process also means countries themselves can better “know their epidemic” enabling them to target their strategic response accordingly.
Much progress to report: UNGASS 2008

Feature Story
51st session of the Commission on Narcotic Drugs
11 March 2008
11 March 2008 11 March 2008
United Nations Office on Drugs and Crime
The Commission on Narcotic Drugs (CND) met for its 51st session in Vienna 10-14 March 2008. At this session governments assessed the progress achieved in meeting the goals defined in 1998 UN General Assembly Special Session on Illicit Drugs.
UNAIDS delivered a statement to the 51st session of the Commission on Narcotic Drugs (Vienna, 10-14 March), calling for increased investment and programmatic action to provide prevention, treatment and support and to protect the human rights of people who use drugs. Read the UNAIDS statement.
The findings of the session will serve as a report to the UNGASS in 2009, together with an identification of future policy-oriented priorities and further actions beyond 2009.
Monitoring the outcome of the 1998 General Assembly Special Session on countering the world drug problem
The 1998 UN General Assembly Special Session on Illicit Drugs (UNGASS) concluded with member nations adopting a political declaration where ambitions and actions for the next decade were outlined. Among other things the declaration said that participating governments commit themselves to “achieving significant and measurable results in the field of demand reduction by the year 2008”.
The United Nations has started a process to assess the results of the international efforts to combat illicit drugs and to review the international conventions on narcotic drugs. The process will be completed with a special session of the UN General Assembly (UNGASS) in New York in 2009.
51st session of the Commission on Narcotic Drugs
Related

Feature Story
Lions Club International signs Central America agreement with UNAIDS and UNICEF
10 March 2008
10 March 2008 10 March 2008
Regional Director of UNAIDS, César Núñez addressed the ceremony of the signing of the cooperation agreement
Credit: UNAIDS/Lions Club
UNAIDS and UNICEF Regional Support Teams (RST) for Latin America have signed a cooperation agreement with the Lions Club International (LCI) in Central America.
The cooperation agreement will allow the organizations to join forces in response to HIV with the goal of promoting universal access to HIV prevention, treatment and care as well as sexual health education and information initiatives in Central America.
The letter was signed by the Regional Director of UNICEF for Latin America and Caribbean, Mr Nils Kastberg; Regional Director of UNAIDS for Latin America, Dr. César Antonio Núñez; and President of the Governor Council Lion’s Club Multiple District D Istmania, PDG Ricardo Domínguez Posada.
The President of the LCI, H.L. Mahendra Amarasurya´s, emphasized the important role that LCI can have to “combine efforts with the United Nations in order to improve the quality of life of the boys, girls, men and women living with HIV, as well as to increase efforts in order to limit the progress of this disease”.
According to Regional Director of UNAIDS, César Núñez, “joint initiatives like this one, with important allies of civil society like the Lions Club, are what is needed in our country in order to keep moving ahead towards the goal of universal access”.

Lions Club International has 1.4 million members in 202 countries around the world
Credit: Lions Club International
Núñez said that “Latin America has been able to develop good practices in relation to the HIV response which will be strengthened by committed parties such as civil society, Governments and international organizations working together.”
The next step will be to prepare a Letter of Agreement among the three organizations and a strategic plan in order to define priorities and concrete actions to be developed in the future months. The plan will elaborate on activities along with estimated budget, a division of labour agreement and the different responsibilities and resources of the signing organizations.
The ceremony took place on 21 February 2008 during the XXXVII Lions Forum for Latin America and the Caribbean in El Salvador and was attended by six Lions Governors from Central American countries.
About Lions Club International
The Lions Club was started by business man Melvin Jones in Chicago in 1917. Jones believed that business should look than narrow professional interests to the progress of their communities and the world. Jones' personal code, "You can't get very far until you start doing something for somebody else," reminds many Lions of the importance of community service.
Today, LCI is the world's largest secular service organization with over 44,500 clubs and more than 1.4 million members in 202 countries around the world. It has been collaborating with the United Nations since 1945.
Related

Feature Story
Abataka: Women shape their future
07 March 2008
07 March 2008 07 March 2008International Women’s Day on 8 March is a global day of celebration to inspire women and celebrate their achievements. The theme of 2008 is “Investing in Women and Girls”. UNAIDS Special Representative Mary Fisher and the women artisans of the Lusaka Zambia jewellery project believe income generation projects are critical in empowering women living with HIV, enabling them to shape their future.
Employment to empower HIV positive women

UNAIDS Special Representative Mary Fisher with the artisans of the Lusaka Jewellery project.
Credit: Mary Fisher
As an advocate for those who share her HIV positive status, UNAIDS Special Representative Mary Fisher has had the opportunity to travel and meet women around the world. Listening to them she saw how employment could contribute to empowering women living with HIV, especially those living in poverty.
“Everywhere I’ve travelled,” she said, “I’ve seen women struggling to sustain themselves and their families against the burden HIV places on them”.
So in 2006 Ms Fisher began to explore sustainable income-generation ideas through which HIV positive women in Zambia and Rwanda could earn a living, care for their families and sustain their health. She met and talked with women to find a product they could make with the traditional skills they already had or could learn which could be sold at a profit.

In Lusaka Zambia the traditional craft of bead-making is getting a modern twist.
Credit: Mary Fisher
The idea of modern fashion jewellery created with the traditional craft of beading which could be promoted and sold by the internet including on Mary Fisher’s website was born.
Making an impact
Today, the jewellery project is thriving. Around 150 HIV positive women who participate in support groups at the Centre for Infectious Disease Research in Zambia (CIDRZ), a Lusaka-based HIV/AIDS research, treatment and care facility; or who live in the local Chikumbuso community, have been trained and employed as jewellery makers.

Modern jewellery designs by artist Mary Fisher are crafted by bead-crocheting.
Credit: Mary Fisher
Mary Fisher is an international artist and the combination of her modern jewellery designs with the women’s traditional craft of bead making is proving a success. The artisans practice the intricate art of bead-crocheting with materials and gemstones and sales are going well. Nearly three dozen varieties of bracelets, designed by artist Mary Fisher, are now in production, with more items on the drawing board. The women are paid for each piece they create and additional profits are invested to buy more materials and employ more women.
Fisher has named some of the jewellery pieces the Abataka Collection as Abataka means “family, community, belonging”.
“I can give my children an education”
On any given day, several dozen women artisans may gather to work on the jewellery and share life stories.
Esther’s story is typical: She contracted HIV from her husband, passed it unknowingly to four of their five children and, since her husband’s death in 2005, raises her family alone.
Before, Esther and her children often skipped doses of HIV medication because they had no food to take them with. Now, they have money for housing, for food – even for books and fees, to keep the children in school. “I am very grateful to God for the beading project so I can give my children education,” she said.
When Esther and her co-workers finish each bracelet, they mark it with a tag to which they sign their names. The signed handiworks make the bracelet makers and bracelet buyers feel like allies across the globe responding to AIDS. One group of buyers in Arizona sent photographs of themselves wearing their bracelets to the Zambian artisans with personal letters of thanks and encouragement.
Looking to the future
With their new earnings and skills from the jewellery project, HIV-positive women artisans aren’t just living day to day, but looking to the future. Many have been able to open savings accounts, start their own small businesses, and obtain better housing for their families.
Ms Fisher calls it the very definition of “the spirit of Abataka: people from around the world befriending African women on their journey. With the world’s help, these women are looking towards a brighter future".
Abataka: Women shape their future
Feature stories:
UNAIDS Special Representative Mary Fisher visits Zambia (29 August 2007)
Press centre:
World renowned artist, author and activist Mary Fisher accepts appointment as UNAIDS Special Representative (18 May 2006)
External links:

Feature Story
International Women's Day 2008: Investing in women and girls
07 March 2008
07 March 2008 07 March 2008
International Women’s Day is celebrated internationally each year on 8 March. While this date has been celebrated since the early 1900s, the United Nations has observed March 8th as International Women’s Day since 1977 to highlight that active participation, equality and development of women is necessary for securing peace, social progress and human rights; and to acknowledge the contribution of women to the strengthening of international peace and security.
This year’s theme is “Investing in Women and Girls” with a strong focus on country level financing for gender equality. Highlighting this issue, the priority theme of the 52nd session of the Commission on the Status of Women (CSW), which concluded 7 March 2008, was “Financing for gender equality and the empowerment of women”.
Commission on the Status of Women
The report of the Secretary-General on financing for gender equality and the empowerment of women (E/CN.6/2008/2) guided the work of the Commission. It identifies and discusses key issues in financing for gender equality and the empowerment of women and suggests policy recommendations.
UNAIDS delivered a statement to the CSW to draw attention to the links between gender inequality and increased vulnerability to HIV infection among women and adolescent girls and to call for ensuring greater and more sustainable financing for gender equality.
Read full UNAIDS statement
Unite to end violence against women
UN Secretary-General Ban Ki-moon opened the 52nd session of the CSW on 25 February 2008 by launching a multi-year campaign to end violence against women that will continue until 2015, to coincide with the target date for the Millennium Development Goals.

The campaign, which will run until 2015, aims to mobilize public opinion to ensure that policy makers at the highest level work to prevent and eradicate violence against women and to secure political will and increased resources to combat the problem.
"Violence against women is an issue that cannot wait. A brief look at the statistics makes it clear. At least one out of every three women is likely to be beaten, coerced into sex or otherwise abused in her lifetime. No country, no culture, no woman young or old is immune to this scourge
It is a campaign for the women and girls who have the right to live free of violence, today and in the future. It is a campaign to stop the untold cost that violence against women inflicts on all humankind."
- Remarks by Secretary General Ban Ki-moon to the Commission on the Status of Women, New York, 25 February 2008.
The campaign aims to mobilize public opinion to ensure that policy makers at the highest level work to prevent and eradicate violence against women and to secure political will and increased resources to combat the problem.
The initiative will harness the existing momentum in the General Assembly and the Security Council to take action against all forms of violence against women, including rape in conflict and post-conflict situations and builds upon decades of work by women activists, women’s groups and other civil society organizations who continue to lead the struggle to expose and counter violence.
Global Coalition on Women and AIDS (GCWA)

The Global Coalition on Women and AIDS.
To mark International Women's Day, the Global Coalition on Women and AIDS wil feature interviews with selected women who have demonstrated extraordinary success in overcoming challenges in their work on HIV. The theme of the interview series is "Victories of Women in HIV and AIDS" and will highlight how women have transformed the art of possibility into a living example of leadership.
Visit the web site of the GCWA to find out more
International Women's Day 2008: Investing in wome
Partners:
Global Coalition on Women and AIDS
External links:
Unite to end violence against women
UN action against sexual violence in conflict: "Stop rape now"
UNIFEM's say NO to violence against women campaign
Publications:
UNAIDS statement to 52nd session of the Commission on the Status of Women
UN action against sexual violence in conflict
Report of the UN Secretary-General on Financing for gender equality and the empowerment of women

Feature Story
Partnerships and linking for action
06 March 2008
06 March 2008 06 March 2008
The Global Health Workforce Alliance (GHWA) held the first ever Global Forum on Human Resources for Health in Kampala, Uganda from March 2-7, 2008. The GHWA, hosted and administered by the World Health Organization (WHO), has been created to identify and implement solutions to the health workforce crisis.
UNAIDS Executive Director Dr Peter Piot gave the following plenary speech on "Partnerships and linking for action".
Plenary speech by Dr Peter Piot, UNAIDS Executive Director
Kampala, Uganda 5 March, 2008.

UNAIDS Executive Director Dr Peter Piot addressing plenary at the Global Forum on Human resources for Health, in Munyonyo, Kampala, 5 March 2008
Credit: UNAIDS/C. Opolot
Thank you Sigrun – and thank you for inviting me here today.
I came to Kampala for three reasons. Firstly, this Forum is one of the most important meetings in public health to take place this year. We are starting to build a coalition to address one of the greatest obstacles to health.
Secondly, I am here to pledge the firm support of UNAIDS to this initiative.
Thirdly, it is time to de-polarize this debate. Whether we invest in the AIDS response or in strengthening health systems. It is not a question of one or the other. Even when it comes to AIDS, it is not simply a question of strengthening health services but also community mobilization. We must not forget about people or health outcomes
The issue of human resources for health is complex. But we all know it’s not a new one. I lived it myself in the mid-70s in rural Zaire. But nor is it limited to Africa. Last week I was in India where this is an enormous maldistribution of human resources for health.
The shortage results from decades of under-investment by governments, donors and international agencies. It has been intensified by globalization, but globalization may also bring some of the solutions. Responsibility for the current situation is shared – between donors, national governments, NGOs, research organization and international organizations among others. We therefore have a shared duty to address it. That’s why this afternoon’s panel, with its focus on partnership, is so vital.
The debate we are having now is long overdue. And a major reason for its happening at all is AIDS!
One of the peculiar characteristics of AIDS is that it exposes injustices. AIDS - more than any other issue - has thrown a spotlight on the urgent need to strengthen human resources for health, for three reasons. Firstly, AIDS represents a significant burden on health systems. In some countries, half of all hospital beds are occupied with patients with AIDS-related illnesses. Secondly, to expand ART, and to make ART sustainable, we need strong health systems. Thirdly, being a health worker does not protect you from becoming infected. Botswana, for example lost approximately 17% of its healthcare workforce to AIDS between 1999 and 2005.
There have been good examples of how AIDS investment has helped overcome the human resources for health crisis. I remember well going to Malawi in 2004 with Sir Suma Chakrabarti, then Permanent Secretary of the UK’s DFID. AIDS had brought the health workforce literally to its knees. There was no way it could cope. It was an emergency that required exceptional measures. DFID and other donors financing the sector agreed to fund a groundbreaking initiative, the Emergency Human Resources Programme, to top up salaries for nurses and other health care workers as an incentive not to leave the country. This was totally novel: donors usually resist paying salaries, but in this case we managed to break the taboo. I’m glad to say that the Global Fund to Fight AIDS, Tuberculosis and Malaria is now supporting this programme.
This is just one example of another characteristic of AIDS: it forces us to do things differently. WHO’s “Treat, train, retain” initiative for health-workers with HIV is another new and pragmatic approach. I don’t know of any other programme that starts by addressing the health of the workers involved. It provides wins all round – to the health workers themselves, to the people who need their services, and to the health sector as a whole. So, when we are talking about strengthening health systems, let’s first make sure that people stay alive! But good partnerships require more than processes. There are too many partnerships that are not enough about results and outcomes.
AIDS funding and programming enhanced essential infrastructure for health facilities. Where HIV services have been integrated into existing service delivery sites, AIDS money helped renovate health facilities, upgraded clinics and laboratories and provided training opportunities for health care workers.
AIDS has also helped promote “task shifting”, an old concept/idea in public health – moving responsibility for certain tasks to other health-workers and community members to free up doctors and nurses to take care of other patients and to deliver other essential health services. Here in Uganda, there is an increasing trend for people living with HIV to take on tasks such as counseling for testing, adherence support, treatment literacy and to produce good quality outcomes. In Kenya, several organizations have been implementing prevention, treatment literacy and home based care programmes, which are led by people living with HIV at the community level. Women Fighting AIDS in Kenya, supported by UNICEF in Kisumu and Port Reitz General Hospitals, trained PLWHAs who were then used as PMTCT champions to provide counseling to ante-natal mothers and their partners.
We also see, particularly here in Africa that faith-based organizations play a major in the fight against AIDS providing vital HIV care and treatment services. For example, Christian hospitals and health centers are providing about 40% of HIV care and treatment services in Lesotho and almost a third in Zambia. In other countries, the formal and informal private sector is also very important.
AIDS has brought in new resources, to benefit not only HIV programmes but health systems more widely. Take for example the Haitian “accompagnateurs” – community workers who have been brought into the health workforce through the AIDS programmme. Or in Rwanda, HIV treatment and care was integrated with regular health services, resulting in better coverage for maternal and child health according to a study by Family Health International (FHI) presented at last year’s PEPFAR Implementers Meeting in Kigali. Les Mutuelles de Santé is another example of financing scheme to mobilize resources for health services.
So I have serious issues with the current wave of statements like “There’s too much money going to AIDS” or “Donors should prioritize health system strengthening”. They completely ignore the growing body of evidence that AIDS expenditure strengthens the health sector and contributes to broader development programmes, besides the fact that AIDS programmes are having measurable results, saving millions of lives. Indeed, AIDS has been an advocate for health systems strengthening.
They also seem to assume that dealing with HIV is mostly about treatment. It isn’t! For every one person we put on antiretroviral therapy, another four or five become infected with HIV. If we don’t radically enhance HIV prevention, demands for treatment will just keep on growing, placing an even greater burden on health systems in the future.
And prevention – except for PMTCT – is far more than a health issue. Prevention is a community based action. Effective HIV prevention derives from a range of multi-sectoral interventions (governments, nongovernmental organizations, faith-based organizations, the education sector, media, the private sector and trade unions and people living with HIV).
A lot of the recent surge of funding started as a direct consequence of the AIDS epidemic. AIDS advocacy did not only succeed in mobilizing money, but it also highlighted the profound disparities in health services that separated the developing countries from the developed world. It is however true that there are examples where AIDS related activities and AIDS funding are taking away health workers from other tasks. AIDS funding created new and more interesting job opportunities for doctors and nurses with NGOs and foreign aid agencies and thus can be a drain on the public sector. We have seen it happening in Malawi and in Zambia where focus of disease programmes shifted to HIV. However, and certainly in the heavily affected countries, the AIDS burdens for health services is also a reality. We need to find common solutions and ways of working together.
This brings me to my next point. AIDS has taught us about the critical value of partnerships. Tackling AIDS is one of the toughest challenges the world faces today. Like dealing with climate change, it’s tremendously complex - way beyond the capacity of any single sector or institution. It’s one of those issues that jolts us out of our comfort zone, and forces us to create new alliances with a variety of constituencies – across sectors and at state and non-state level.
UNAIDS itself is a joint programme. We are working with a wide range of constituencies – government, scientists, business, labour, and the media. One of the most important partnerships of all has been our relationship with civil society. It was the activists who kick-started the AIDS movement. Without them, we wouldn’t have achieved anything like the progress we’ve made. It’s thanks to these partnerships that we have been able to mobilize political momentum around AIDS, to leverage funding to $10 billion per year.
In the twelve years since UNAIDS was created, we’ve learnt a lot about partnerships. We’ve seen the advantages of being able to convene diverse actors from public, private, and non profit sectors – all with different strengths. They have the potential to achieve spectacular results – way beyond anything they could hope to achieve on their own.
But coordination and accountability are still important. That’s why UNAIDS established the Three Ones principles, as a framework for partnerships on AIDS. Just to remind you, these are: one agreed national action framework, one national coordinating authority and one agreed monitoring and evaluation system.
The lessons we’ve learnt through implementing the Three Ones are salutary – and very relevant to the aims of this Alliance. The Agenda for Action is right to highlight the need for “national responses to be guided by a national leadership that convenes all actors around one agreed national effort”, and to point to the importance of accountability. The challenge is to engage serious commitment at all levels – in-country, in donor capitals and international organization headquarters. This requires time and effort. But it will be time and effort well spent.
I began today by saying that addressing the shortage of human resources for health was a joint responsibility. It is something that no institution can tackle alone. It is complex, cross-sectoral and long-term. And, like AIDS, it is not a quick-fix problem and there is no one solution that fits all. This may be a major reason why so little has been done before. Another reason may be the fact that the current crisis of human resources for health is also a highly political issue and therefore any possible solutions need to have full political support. But coming together in this alliance is in itself a tremendous step forward. There is a lot at stake; therefore our response must address the emergencies of today and to draw up longer-term plans for the future.
The Agenda for Action offers a comprehensive menu of activities, but I want to suggest some very concrete actions where we can all work and benefit together.
The first is that we must build partnerships far beyond the public sector. Partnerships are crucial for the success of any solution. We must also look at the critical role of non-state actors in the provision of services and their role in the training of human resources. In many countries, 40 to 60% of health services are delivered by the private sector. We have to establish more private/public partnerships with greater engagement of the private sector, beyond workplace programmes. Equally, in many countries, particularly here in Africa, many clinics and health centers are run by faith-based organization. We need to bring them all into the policy dialogue of heath services provision.
The second is to engage the full participation of civil society. As I mentioned earlier, civil society has been at the heart of the AIDS response from the very beginning. And its presence there has been vital. Not only does civil society activism mobilize action, but community members are an invaluable source of knowledge about what works and about how to reach people. We must listen and learn from them, and at the same time invest in building their capacity to deliver alongside that of public sector.
The third is for health ministries to make improving human resource management a priority. This is implicit in the Agenda for Action. But I think we need to spell it out more clearly. Today’s crisis has come about for two reasons. Lack of investment and lack of management. There’s a lot to do, but one of the first steps should be to establish incentives for performance and raise health-worker morale.
Fourth, we need to work together to question and challenge our concepts of fiscal space, predict medium term expenditure frameworks and the suitability of salary supplementation. We have to involve ministries of finance in the discussions of solutions. We should also work together with the World Bank and IMF on these constraints.
There is also the need to address the issue of public sector pay and work conditions. To address issues such as poor infrastructure, lack of equipment and drugs, long hours and heavy workloads and lack of career development in addition to poor remuneration. This needs to be combined with putting human resources for health on the agenda of civil service reform and donor willingness support and invest in supplementing health workers’ salaries and training. Donors and countries should consider the lessons learnt from the Malawi experience.
These issues are at the heart of any assessment of countries’ ability to scale up the response and the achievements of the MDGs. They are relevant for all of the health MDGs and need close examination and a common assessment of the risks and opportunities.

Press conference at the close of Global Forum on Human resources for Health, Kampala 5 March, 2008. (From left): Chair of the board Global Health Workforce Alliance, Dr Lincoln Chen; UNAIDS Executive Director, Dr Peter Piot and Representative of Women Living with HIV in Uganda, Beatrice Were.
Credit: UNAIDS/C. Opolot
We can be very ambitious, but need clear targets, goals and a partnership, where put the institutional interest aside. Fight for common good and common goal. We need to re-set the rules and to put into practice what has been discussed globally at country level. Every research programme must include overhead (/indirect costs for strengthening capacity. This is starting to be done among largest investors in heath (GAVI; GF; PEPFAR etc).
We also need to find a practical way to compensate low and middle-income countries that are losing their skilled staff in whose education they have invested.
The final – and most relevant for this afternoon’s session - is to be serious about applying the Three Ones principles, for all parties to come together and align around a single strategic plan for strengthening human resources for health that focuses clearly not just on process but on results. One National AIDS Coordination authority and one agreed country-level monitoring and evaluation system. Such a framework has been invaluable for a well coordinated AIDS response. We are not there yet, but we have made progress.
If we make progress on action plan, it will be because have worked together. It is through diversity we will success. Pragmatic approach is needed, one step at a time, and strong leadership which will hold us together. I believe we have that leadership.
That may sound ambitious. But if we can come back in a year’s time and say we’ve made progress in these four areas, the world’s health workforce will look a lot more robust than it does today – and its population will be fitter as a result.
We have to act now and “to work together to ensure access to a motivated, skilled, and supported health worker by every person in every village everywhere.” Dr. LEE Jong-wook
Thank you.
Partnerships and linking for action
Cosponsors:
WHO - Global Health Workforce Alliance
Feature stories:
Health workforce crisis limits AIDS response (29 February 2008)
External links:
First Global Forum on Human Resources for Health 2-7 March 2008, Kampala, Uganda
Publications:
Scaling up access to HIV prevention, treatment, care and support: The next steps (UNAIDS, 2006)
Global Health Workforce Alliance Strategic Plan (WHO)
Related

Feature Story
Uniting against female genital mutilation
04 March 2008
04 March 2008 04 March 2008
The Interagency Statement on
Eliminating Female genital
mutilation was signed by
UNAIDS, UNDP, UN Economic
Commission for Africa, UNESCO,
UNFPA, the Office of the High
Commissioner on Human Rights,
UNHCR, UNICEF, UNIFEM and
WHO.
Reaffirming their commitment to the elimination of female genital mutilation, 10 United Nations bodies including UNAIDS, have expressed their commitment to support governments, communities, and women and girls to abandon the practice within a generation.
There are a growing number of examples in countries around the world where the prevalence of female genital mutilation has declined. The interagency statement “Eliminating female genital mutilation” is a joint initiative to support the scaling up of good examples to become common practice.
Female genital mutilation, also called female genital cutting and female genital mutilation/cutting, violates the rights of women and girls to health, protection and even life as the procedure sometimes results in death. Although decades of work by local communities, government, and national and international organizations have contributed to reducing the prevalence of female genital mutilation in many areas, the practice remains widespread.
Damages public health and human rights
Between 100 and 140 million women and girls in the world are estimated to have undergone female genital mutilation and 3 million girls are estimated to be at risk of undergoing the procedures every year.
The statement points out that female genital mutilation is a manifestation of unequal relations between women and men with roots in deeply entrenched social, economic and political conventions.
The practice is believed to enhance a girl’s chastity and chances of marriage by controlling her sexuality. As such, it not only infringes on women’s sexual and reproductive health; it also perpetuates gender roles detrimental to women.
"We recognize that traditions are often stronger than law, and legal action by itself is not enough,” said all the agencies involved. “Change must also come from within. This is why it is critical for us to join hands and work closely with communities and their leaders so that they can bring about sustainable social change.”
Health complications of female genital mutilation
The statement highlights the damaging effect of female genital mutilation on the health of women, girls and newborn babies. Immediate risks of the practice include severe pain, shock and even death through hemorrhaging. The use of the same surgical instrument without sterilization could increase the risk for transmission of HIV between girls who undergo female genital mutilation together.
Long-term health risks include chronic pain, reproductive tract infections, birth complications and psychological consequences. An increased risk for bleeding during intercourse may increase the risk for HIV transmission. The increased prevalence of herpes in women subjected to female genital mutilation may also increase the risk for HIV infection, as genital herpes is a risk factor in the transmission of HIV.
The UN said, “We are becoming increasingly concerned about the medicalisation of female genital mutilation. This is where the mutilation is performed by health professionals in health facilities. The argument that a mild form performed by medically trained personnel is safer is commonly heard in countries where female genital mutilation is practiced. But this should never be considered as an option."
The statement argues that the treatment and care of the adverse health consequences of female genital mutilation should be an integral part of health services, such as safe motherhood and child survival programmes, sexual health counselling, psycho-social counselling, prevention and treatment of reproductive tract infections and sexually transmitted infections including HIV and AIDS, prevention and management of gender-based violence, youth health programmes and programmes targeting traditional birth attendants (who may also be traditional circumcisers).
New evidence and lessons learned
The interagency statement is based on new evidence and lessons learnt over the past decade. It highlights the wide recognition of the human rights and legal dimensions of the problem and provides current data on the prevalence of female genital mutilation.
It summarizes findings from research on the reasons why the practice continues, highlighting that the practice is a social convention which can only be changed through coordinated collective action by practising communities. It also summarizes recent research on its damaging effects on the health of women, girls and newborn babies.
Drawing on experience from interventions in many countries, the new statement describes the elements needed, for both working towards complete abandonment of female genital mutilation, and caring for those who have suffered, and continue to suffer, from its consequences.
The Interagency Statement on Eliminating Female genital mutilation was signed by the Joint UN Programme on HIV/AIDS (UNAIDS), the UN Development Programme (UNDP), The UN Economic Commission for Africa (UNECA), the UN Educational, Scientific and Cultural Organizations (UNESCO), the UN Population Fund (UNFPA), the Office of the High Commissioner on Human Rights (UNHCHR), The UN Refugee Agency (UNHCR), UNICEF, the UN Development Fund for Women (UNIFEM) and the World Health Organization (WHO).
Uniting against female genital mutilation
Cosponsors and partners:
UN Development Programme (UNDP)
UN Economic Commission for Africa (UNECA)
UN Educational, Scientific and Cultural Organizations (UNESCO)
UN Population Fund (UNFPA)
The Office of the High Commissioner on Human Rights (UNHCHR)
UN Refugee Agency (UNHCR)
United Nations Children Fund (UNICEF)
UN Development Fund for Women (UNIFEM)
World Health Organization (WHO)
External links:
WHO Department of Reproductive Health and Research
Publications:
Eliminating Female genital mutilation. An interagency statement. (February 2008)

Feature Story
HIV-related travel restrictions
04 March 2008
04 March 2008 04 March 2008Since the beginning of the HIV epidemic, governments and the private sector have implemented travel restrictions with regard to HIV positive people wishing to enter or remain in a country for a short stay (e.g. business, personal visits, tourism) or for longer periods (e.g. asylum, employment, immigration, refugee resettlement, or study).

The international task team on HIV-relate
travel restrictions met for the first time in
Geneva on 25-26 February. The meeting
was co-chaired by UNAIDS and the
Government of Norway.
UNAIDS has set up an international task team to heighten attention to the issue of HIV-related travel restrictions (both short-term and long-term) on international and national agendas and move towards their elimination.
The international task team met for the first time in Geneva on 25-26 February. The meeting, co-chaired by UNAIDS and the Government of Norway, brought together representatives of governments, inter-governmental organizations and civil society, including the private sector and networks of people living with HIV.
According to data collected by the European AIDS Treatment Group, a total of 74 countries have some form of HIV-specific travel restrictions, 12 of which ban HIV positive people from entering for any reason or length of time. The most common reasons used are to protect public health and to avoid possible costs associated with care, support and treatment of people living with HIV.
Whatever the reason, HIV-related travel restrictions raise fundamental issues regarding the human rights of non-discrimination and freedom of movement of people living with HIV in today’s highly mobile world.
In the year 2000, the World Tourist Organization estimated that there were 698 million international arrivals world-wide. The majority of these people are travelling for short periods of time, e.g. for tourism, business, conferences, family visits. With regard to longer-term mobility, the International Organization for Migration (IOM) estimates that some 175 million migrants currently live and work outside their country of citizenship, i.e., 2.9 per cent of the world’s population.
HIV -related travel restrictions usually take the form of a law or administrative instruction that requires people to indicate their HIV-free status before entering or remaining in a country. Some countries require people to undergo an HIV test whereas others require an HIV-free certificate or simply that people declare their HIV status.
Testing under such circumstances is akin to mandatory testing, and in many instances is done without appropriate pre and post-test counselling or safeguards of confidentiality. Any HIV testing should be done voluntarily and on the basis of informed consent.
The personal impact of HIV-related travel restrictions can be devastating for the individual seeking to immigrate, to gain asylum, to visit family, to attend meetings, to study, or to do business. The candidate immigrant, refugee, student or other traveller may simultaneously learn that s/he is infected with HIV, that s/he may not be allowed to travel, and possibly that his/her status has become known to government officials, or to family, community, and employer, exposing the individual to possibly serious discrimination and stigma.
For those already in a receiving country, they may face summary deportation without due process of law and protection of confidentiality. Under such circumstances, there is every incentive to hide or deny one’s HIV status and to avoid contact with immigration authorities and health care workers. Both immigration controls and public health efforts are thereby undermined, while individuals are cut off from prevention, assistance and, perhaps, needed health services.

“Travel restrictions based on HIV status
again highlight the exceptionality of AIDS,
especially short-term restrictions," said
UNAIDS Executive Director Dr Peter Piot.
“Travel restrictions based on HIV status again highlight the exceptionality of AIDS, especially short-term restrictions," said UNAIDS Executive Director Dr Peter Piot. "No other condition prevents people from entering countries for business, tourism, or to attend meetings. No other condition has people afraid of having their baggage searched for medication at the border, with the result that they are denied entry or worse, detained and then deported back to their country", he added.
While recognizing that control of a country’s borders and matters of immigration fall under the sovereign power of individual States, national laws and regulations should ensure that people living with HIV are not discriminated against in their ability to participate equally in international travel, in seeking entry or stay in a country that is not their own. The International Guidelines on HIV/AIDS and Human Rights state that any restriction on liberty of movement or choice of residence based on suspected or real HIV status alone, including HIV screening of international travellers, is discriminatory.
Besides being discriminatory, travel restrictions have no public health justification. HIV should not be considered to be a condition that poses a threat to public health in relation to travel because, although it is infectious, the human immunodeficiency virus cannot be transmitted by the mere presence of a person with HIV in a country or by casual contact.
Restrictive measures can in fact run counter to public health interests, since exclusion of HIV-positive non-nationals adds to the climate of stigma and discrimination against people living with HIV, and may thus deter nationals and non-nationals alike from coming forward to utilize HIV prevention and care services. Moreover, travel restrictions may encourage nationals to consider HIV a “foreign problem” that has been dealt with by keeping foreigners outside their borders, so that they feel no need to engage in safe behaviour themselves.
Travel restrictions do not have an economic justification either. People living with HIV can now lead long and productive working lives, a fact that modifies the economic argument underlying blanket restrictions: concern about migrants’ drain on health resources must be weighed with their potential contribution. Furthermore, the continued expansion of treatment programmes towards Universal Access by 2010, and the sustained fall in the cost of treatment in low- and middle-income countries dispels the myth that the travel of a HIV positive person would drive up the cost of health care systems abroad.
The creation of an International Task Team on HIV-related Travel Restrictions is a critical opportunity to heighten attention to the issue of HIV-related travel restrictions on international and national agendas and move toward their elimination.
Developing Specific Recommendations for Positive Change

The Task Team will generate concrete
recommendations on specific actions that
different stakeholders can take to move
towards the elimination of HIV-related
travel restrictions.
The International Task Team comprises two working groups which focus on short-term and long-term restrictions, supported and guided by a Steering Committee. The Working Groups and the Steering Committee of the Task Team will meet four times before August 2008, when its final recommendations will be presented at the International AIDS Conference in Mexico. The Task Team will generate concrete recommendations on specific actions that different stakeholders (government, civil society, intergovernmental organizations and the private sector) can take to move towards the elimination of HIV-related travel restrictions. The Task Team will focus on key strategic actions that:
- Increase attention to the issue of HIV travel restrictions internationally, regionally and nationally
- Influence governments that have HIV-related travel restrictions relating to entry and short-term stay to remove such restrictions; and
- Spur longer term action to move towards the elimination of all HIV-specific travel restrictions.
In developing these recommendations, the Steering Committee will be supported by the Working Groups which will be requested to undertake:
- A critical mapping of the current situation related to the use of short-term and long-term HIV-related travel restrictions
- An analysis of the obstacles to eliminating the different types of HIV-related travel restrictions and possible actions by various national and international stakeholders to support this elimination, and
- Recommendations on best practice regarding the entry and stay of people living with HIV in different contexts of mobility and migration.
Recommendations will support the principles of non-discrimination and the Greater Involvement of People Living with HIV and rational HIV-related policies for travellers, migrants and mobile populations in sending and receiving countries – in the context of efforts to achieve universal access to HIV prevention, treatment, care and support, as agreed by governments at the High Level Meeting on AIDS (2006).
The International Task Team on HIV-related Travel Restrictions will hold its next meeting on 31st March – 2nd April in Geneva.
HIV-related travel restrictions
Feature stories:
Read about the second meeting of the International Task Team on HIV-Related Travel Restrictions (OHCHR web site)
External links:
Global Fund 16th Board Meeting – Acknowledgement of the UNAIDS Commitment to Create a Task Team on Travel Restrictions
IAS Policy Statement
European AIDS Treatment Group
Multimedia:
Listen to Shaun Mellors, Senior Technical Adviser, Human Rights, International HIV/AIDS Alliance
Listen to Gracia Violeta Ross Quiroga, National Chair, Bolivian Network of People with HIV/AIDS
Related

Feature Story
Health workforce crisis limits AIDS response
29 February 2008
29 February 2008 29 February 2008
The Global Health Workforce Alliance (GHWA) is convening the first ever Global Forum on Human Resources for Health in Kampala, Uganda from March 2-7, 2008.
The GHWA, hosted and administered by the World Health Organization (WHO), has been created to identify and implement solutions to the health workforce crisis. What is this crisis and how does it impact on the AIDS response?
Healthcare systems depend on trained staff
One of the major obstacles identified to scaling up access to HIV prevention, treatment, care and support in a country is a weak national healthcare system.
The question of human resources for health is a critical factor in any effective response to AIDS. A shortage of trained health care workers, particularly in low and middle-income countries, presents a real challenge to the ability of a country to respond to the HIV prevention, treatment and care needs of their populations.
In parts of sub-Saharan Africa shortages are so acute that they limit the potential to scale up programmes aimed at achieving health-related Millennium Goals including the roll-out of treatment for AIDS. - World Health Assembly, 2005
WHO estimates that more than 4 million additional doctors, nurses, midwives, managers and public health workers are urgently needed to avert serious crises in health-care delivery in 57 countries around the world—26 of these in sub-Saharan Africa. WHO estimates that at least 1.3 billion people around the world lack access to even the most basic health care.
Insufficient human resources has been identified as a primary obstacle to the delivery of antiretroviral treatment and other HIV-related services in many countries in Eastern Europe, Africa and Asia. Many healthcare systems have poor availability and quality of pre- and post-test counselling, health education, home care, diagnosis and treatment of opportunistic infections.
Governments pledge to increase capacity
At the 2006 High Level Meeting on AIDS, UN Member States reaffirmed their commitment to fully implement the 2001 Declaration of Commitment on HIV/AIDS and further strengthened international commitment on AIDS by:
“Pledging to increase capacity of human resources for health, and committing additional resources to low- and middle-income countries for the development and implementation of alternative and simplified service delivery models and the expansion of community-level provision of comprehensive AIDS, health and other social services.” However translating government commitment to increasing capacity into more health workers on the ground is a challenge of some complexity.
Balancing macroeconomic stability and staff retention
While AIDS funding has increased in recent years, simply pouring this into the healthcare system of a country to strengthen capacity is not the solution.
Most economists agree that a high rate of growth of a money supply causes a high rate of inflation - a rise in the general level of prices of goods and services in a given economy over a period of time.
Governments believe that fiscal and monetary policies – to keep inflation low - are needed to control and manage their economy to prevent potentially damaging sharp shocks and fluctuation in growth.
Low-income countries with high HIV-prevalence have to juggle the need to invest in their healthcare systems with a responsibility to maintain macroeconomic stability – nationally and regionally.
These economic policies include keeping salaries low and so constrain the hiring of the doctors, nurses, community health-care workers. Low salaries lead to low worker morale and low productivity and make it extremely difficult for some countries to retain their staff.
Open labour markets mean skilled professionals are migrating in record numbers to high-income countries, draining human capacity where it is most needed.
Global Forum on Human Resources for Health
Consensus is growing that this is a global crisis which calls for coordinated action.
The Global Health Workforce Alliance (GHWA) has been established to explore and implement solutions to this health workforce crisis. It is hosted and administered by the World Health Organization (WHO).
As a first step in the process, the GHWA are holding the first Global Forum on Human Resources for Health in Kampala this week. This meeting brings together government leaders, health and development professionals, civil society and academics from around the world who hope to consolidate a global movement on this.
Participants will explore solutions to improving education, training, and health sector management as well as looking at recent trends in migration.
Health workforce crisis limits AIDS response
Cosponsors:
WHO - Global Health Workforce Alliance
External links:
First Global Forum on Human Resources for Health 2-7 March 2008, Kampala, Uganda
Publications:
Scaling up access to HIV prevention, treatment, care and support: The next steps (UNAIDS, 2006)
Global Health Workforce Alliance Strategic Plan (WHO)